EBI® OsteoGen™
Surgically Implanted Bone Growth Stimulators

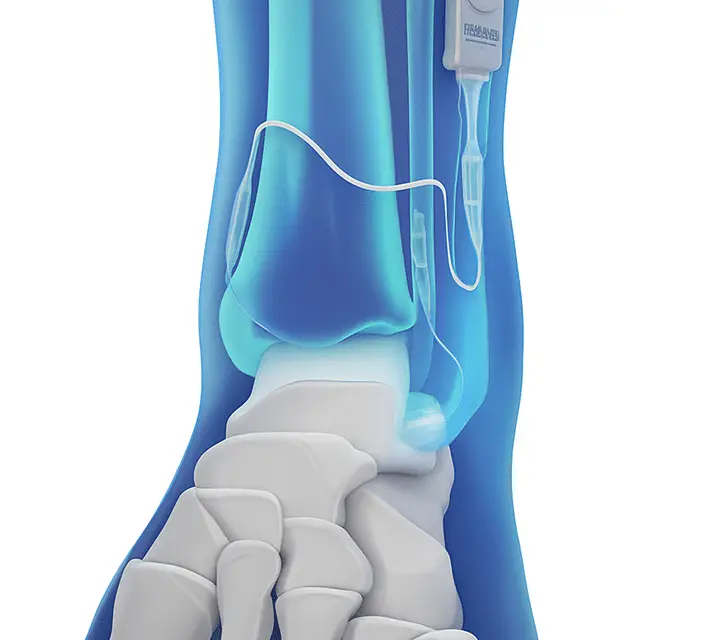
Overview
About the Device
The EBI® OsteoGen™ Surgically Implanted Bone Growth Stimulator is an FDA-approved, class III, surgically implanted, battery-powered direct current (DC) bone growth stimulation device designed to promote bone healing in patients with nonunion fractures. Implanted near the treatment site during the procedure, it provides consistent, around-the-clock therapeutic treatment to increase the probability of a successful healing outcome.

Features & Benefits
The first and only bone growth stimulation implant to support fracture nonunion healing outcomes with no need for patient compliance.
The cathode(s) are placed precisely in and around the targeted treatment site offering controlled treatment directly to the area where patients need it most.
Provides constant, around-the-clock therapeutic treatment providing surgeons and patients the best opportunity for successful healing outcomes.
Powered by pre-clinically proven Direct Current technology
Clinically proven improved clinical outcomes
How the OsteoGen™ Stimulator Works
The OsteoGen™ stimulator delivers safe, effective and clinically proven direct current stimulation through a pair of titanium cathodes. The cathodes are placed in or around the treatment site, and the generator is implanted away from the cathodes during surgery. The area between and around the cathodes safely and effectively stimulates the body’s natural healing mechanisms to promote bone growth.
Clinical Effectiveness & FDA Approval
The OsteoGenTM stimulator is supported by pre-clinical research and published clinical studies demonstrating its safety and effectiveness in improving healing outcomes.
The OsteoGenTM stimulator has demonstrated an overall success rate of 86% for fracture nonunions.1+
Device Specifications
Technology: Direct Current (DC)
Original FDA Approval Date: 1980
Model: OsteoGenTM Single-lead
- Signal: 20 µA
- Cathode length: 8cm
Model: OsteoGenTM Dual-lead
- Signal: 40 µA (20 µA per cathode)
- Cathode length: 8cm

Is the OsteoGen™ Stimulator Right for You?
The OsteoGen™ stimulator is ideal for patients with fracture nonunions who are at risk of delayed bone healing nonunion.
It is prescribed and implanted by orthopedic surgeons as part of a comprehensive treatment plan.
Frequently Asked Questions
Ready to Start Healing?
Ready to support your recovery with the OsteoGen™ stimulator?
INDICATIONS
The OsteoGen™ is indicated in the treatment of long bone nonunions. A nonunion is considered to be established when the fracture site shows no visibly progressive signs of healing.
CONTRAINDICATIONS
There are no known contraindications to the use of this device, however, due to insufficient clinical experience, it is not recommended that it be used in the following conditions: pathological fractures due to malignant tumors or in the presence of active osteomyelitis.
WARNINGS
Long-Term Biocompatibility: While titanium has a clinical history of nearly thirty years of use, longer term effects of implantation in humans are unknown. Titanium does not contain nickel, chromium or cobalt, which have been known to provoke a hypersensitivity response in some patients.
PRECAUTIONS
Electrosurgery
Electrosurgical instruments are capable of producing radio frequency voltages of such magnitude that direct coupling can occur between the cautery tip and lead system of the generator. To preclude the possibility of damage to the generator electronics, electrosurgical equipment should not be used on the patient in the immediate vicinity of the implanted stimulator. If electrosurgery must be done after implantation of the device, leave the electrode/ cathode in place so it remains connected and remove the generator, placing it outside of the body with a gloved hand. Only when electrosurgery is completed, place the generator back subcutaneously into the soft tissue.
Diathermy (Microwave, Shortwave or Ultrasonic)
Therapeutic diathermy should not be used in the treatment of a patient who has an OsteoGen™️ implanted, since this equipment can also produce voltages which may cause damage to the electronics. Diathermy must never be applied over the site of any bone growth stimulator since high currents induced in the electrode lead can cause burning of the tissues in contact with the cathode (electrode tip).
Handling
The energy source and electronics of the generator are heavily protected within the titanium case and will be unaffected by normal handling. However, the possibility of damage by mechanical shock, such as a drop onto a hard floor, cannot be precluded. Any unit subjected to this type of accident should not be implanted. Out of service units should be disposed of by industrial garbage disposal. Do not dispose of any unit in an open fire. Use With Internal/External Fixation: When the Stimulator is used in conjunction with metal internal/external fixation devices, caution must be taken to prevent contact with the cathode and the metallic implant. If the cathode comes into contact with the metallic implant, a current dissipation could occur. This reduction of current density could affect the level of OsteoGen™️ normally expected. Secondly, if the anode was to come into contact with the metal implant, possible corrosion could occur at the site of contact. Use of a Second Stimulator: Two or more treatments have been required in up to 3% of the patients reported in the clinical trials. That is, a complete union may not be achieved with the first Stimulator, necessitating the implantation of a second unit. MRI: The safety and effectiveness of the Stimulator during MRI procedures has not been established. MR imaging at close proximity to implanted devices may be associated with tissue heating, nerve stimulation or movement. Warning: The MR image of the area close to the generator may be distorted.
PATIENTS
If you have additional questions regarding your doctor’s instructions in using this device, or you are experiencing any type of adverse reaction with the use of this device, contact your doctor immediately. For further product information, refer to the Patient Manual PN1067796-00 or contact EBI®️ Customer Care at 800-526-2579.
References:
- Paterson DC, Lewis GN, Cass CA. Treatment of delayed union and nonunion with an implanted direct current stimulator. Clin Orthop Relat Res. 1980 (148) may:117-128.
+ The original clinical study which led to PMA approval in 1980 yielded an overall success rate of 78% SS&ED.

