Proven Technologies for Active Healing.
Explore the largest portfolio of clinically proven solutions engineered to enhance bone healing and improve patient outcomes.

NON-surgical DEVICES
Comfortable, At-Home Therapy Backed by Clinical Evidence

Biomet® SpinalPak®
Non-invasive Spine Fusion Stimulator System
The Biomet® SpinalPak® Non-invasive Spine Fusion Stimulator is a class III, FDA approved, non-invasive bone growth stimulation device designed as an adjunctive therapy to aid in the healing of spinal fusions.
Key Features:
The small, intuitive device encourages patients to treat compliantly on the go while improving their fusion outcomes
The lightweight, flexible, Soft-Touch® electrodes weigh less than an ounce, minimizing unwanted weight around the treatment site
The flexible placement of the Soft-Touch® electrodes optimizes precise stimulation, penetrating evenly across the targeted treatment area
The Soft-Touch® electrodes may be comfortably and discreetly worn under a brace
Backed by statistically significant pre-clinical, scientific research and clinical evidence which demonstrated improved clinical outcomes

Biomet® OrthoPak®
Non-invasive Bone Growth Stimulator System
The Biomet® OrthoPak® Non-invasive Bone Growth Stimulator is a class III, FDA approved, non-invasive bone growth stimulation device designed as an adjunctive therapy to aid in the healing of established nonunions.
Key Features:
The small, intuitive device encourages patients to treat compliantly on the go while improving their healing outcomes
The lightweight, flexible, Soft-Touch® electrodes weigh less than an ounce, minimizing unwanted weight around the treatment site
The flexible placement of the Soft-Touch® electrodes optimizes precise stimulation, penetrating evenly across the targeted treatment area
The Soft-Touch® electrodes may be comfortably and discreetly worn under clothing or a brace
Backed by statistically significant pre-clinical, scientific research and clinical evidence which demonstrated improved clinical outcomes

Biomet® EBI®
Bone Healing System
The Biomet® EBI® Bone Healing System is a class III, FDA approved, non-invasive bone growth stimulation device designed as an adjunctive therapy to aid in the healing of nonunion fractures, failed fusions, and congenital pseudarthrosis.
Key Features:
The only bone growth stimulator with three distinct indications, providing surgeons options to treat various conditions with one clinically proven, safe and effective nonsurgical device
The small, lightweight, easy-to-use device empowers patients to treat compliantly on the go while improving their healing outcomes
The 12 flexible, hand-crafted, therapeutic treatment coil options provide complete, 360-degree coverage around the treatment site
The therapeutic treatment coils may be comfortably and discreetly worn over clothing, bracing, or a cast
Backed by statistically significant pre-clinical, scientific research and clinical evidence which demonstrated improved clinical outcomes
SURGICAL DEVICES
Continuous, Around-the-Clock Stimulation
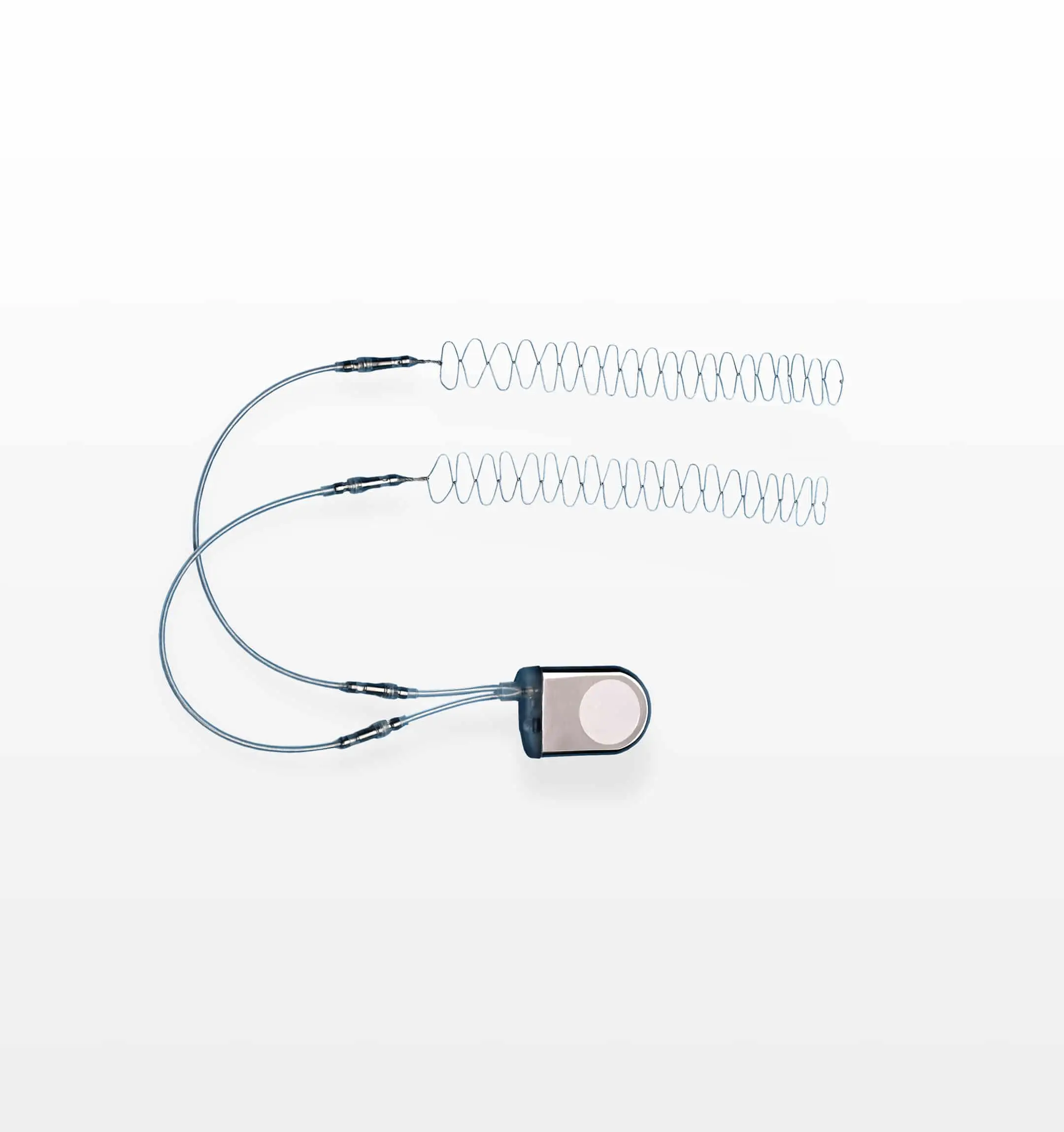
SpF®
Implantable Spinal Fusion Stimulators
The SpF® implantable stimulator is a class III, FDA approved, bone growth stimulation implant designed as an adjunctive therapy to aid in the healing of spinal fusions. It is a proven, safe and effective electrical bone growth stimulation therapy that has improved patient healing outcomes for over 35 years.
Key Features:
The first and only bone growth stimulation implant to support spinal fusion outcomes
The unique cathodes are placed precisely over the targeted treatment site offering controlled treatment to the area where patient’s need it most
The device provides constant, around-the-clock stimulation therapy, empowering surgeons to improve their patient’s healing outcomes with no concern for compliance
The innovative cathode design enables increased surface area for active bone growth stimulation at the targeted treatment site
The sterilely packed implant improves operational efficiencies; no special instrumentation required

EBI® OsteoGen™
Surgically Implanted Bone Growth Stimulators
The EBI® OsteoGen™ implantable stimulator is a class III, FDA approved, implantable bone growth stimulation device designed as an adjunctive therapy to aid in the healing of nonunion fractures. It is a proven, safe and effective bone growth stimulation therapy that has been improving patient outcomes for over 40 years.
Key Features:
The first and only long bone growth stimulation implant to support fracture nonunion healing outcomes
The unique cathodes are placed precisely over the targeted treatment site offering controlled treatment to the area where patient’s need it most
The device provides constant, around-the-clock stimulation therapy, empowering surgeons to improve their patient’s healing outcomes with no concern for compliance
The innovative cathode design enables increased surface area for active stimulation at the targeted treatment site
The sterilely packed implant improves operational efficiencies; no special instrumentation required

