Biomet® OrthoPak®
Non-invasive Bone Growth Stimulator System

Overview
About the Device
Physicians may prescribe a bone growth stimulator to increase the probability of fracture nonunion healing. The Biomet® OrthoPak® Non-invasive Bone Growth Stimulator System is a class III, FDA approved, nonsurgical electrical bone growth stimulation device designed to aid in the healing of fracture nonunions. Powered by Capacitive Coupling (CC) technology, the OrthoPak® stimulator delivers targeted, therapeutic treatment directly to the fracture nonunion, providing patients the best opportunity for successful bone healing outcomes.
Patients recovering from fracture nonunions can benefit from the OrthoPak® stimulator’s convenient home-based treatment, promoting safe and effective bone healing outcomes.

Features & Benefits
Small, non-surgical device encourages patients to treat discreetly on the go
Lightweight and versatile design optimizes patient comfort and wearability
Soft-Touch® electrodes optimize precise stimulation, penetrating evenly across the treatment site
Powered by pre-clinically proven Capacitive Coupling technology
Clinically proven improved clinical outcomes
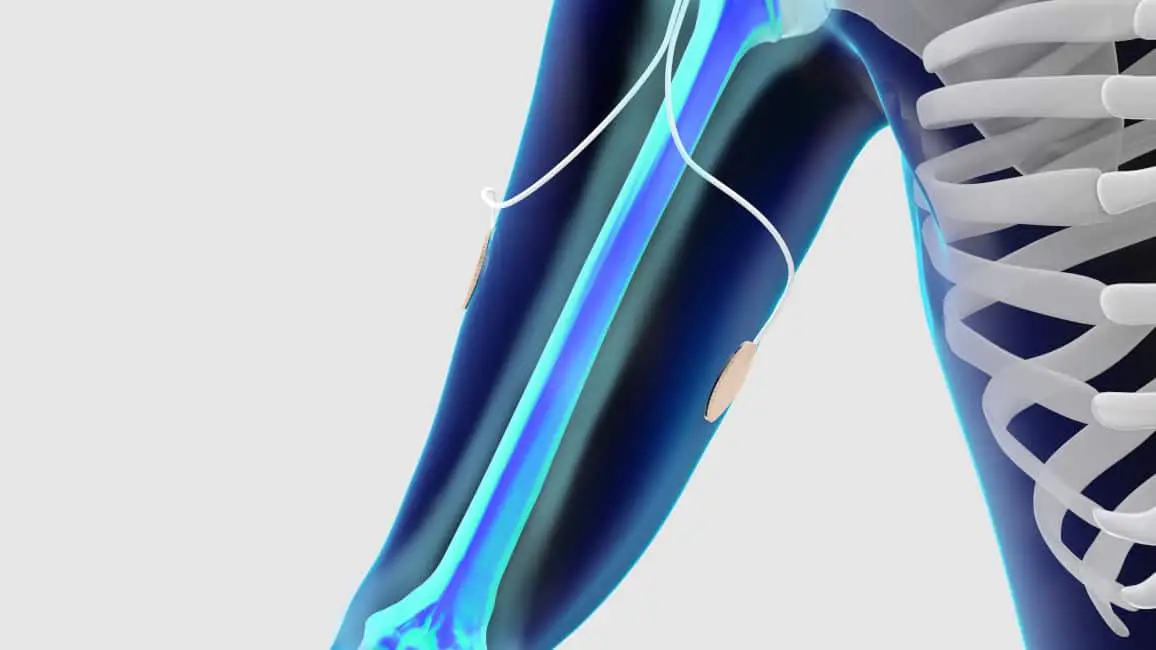
How the OrthoPak® Stimulator Works
The OrthoPak® stimulator delivers safe and clinically proven capacitive coupling stimulation through the Soft-Touch® electrodes. The Soft-Touch® electrodes are placed on each side of the treatment site, 180 degrees apart from one another. The area surrounding the electrodes is stimulated, inducing the body’s natural healing mechanisms to safely and effectively promote bone growth at the targeted treatment site.

How to Use the OrthoPak® Stimulator
The OrthoPak® stimulator is simple to set up and easy to use.
Clinical Effectiveness & FDA Approval
The OrthoPak® stimulator is supported by pre-clinical research and published clinical studies demonstrating its safety and effectiveness in improving bone healing outcomes.
The OrthoPak® stimulator has demonstrated success rates as high as 77%.1+
Device Specifications
Technology: Capacitive Coupling (CC)
Signal: 60kHz sinusoidal wave
Delivery Method: Soft-Touch® electrodes
Device Weight: 3.15 oz.
Original FDA Approval Date: 1986

Is the OrthoPak® Stimulator Right for You?
The OrthoPak® Stimulator is ideal for patients with fracture nonunions.
The device requires a prescription by a licensed physician as part of a comprehensive fracture management plan, and is designed for patients that could benefit from non-surgical adjunctive treatment to support bone healing.
Frequently Asked Questions
Order the OrthoPak® Stimulator Today
Ready to support your recovery with the OrthoPak® stimulator?
INDICATION
The Biomet®️ OrthoPak®️ Non-invasive Bone Growth Stimulator System is indicated for the treatment of an established nonunion acquired secondary to trauma, excluding vertebrae and all flat bones, where the width of the nonunion defect is less than one-half the width of the bone to be treated. A nonunion is considered to be established when there are no visibly progressive signs of healing – P850022/S017.
USAGE
The OrthoPak®️ stimulator is designed to deliver 270 days of continuous therapeutic treatment for 24 hours per day. The recommended daily therapeutic treatment is continuous for 24 hours. Federal Law (U.S.A.) restricts this device to sale by or on the order of a physician. Rx Only – Prescription Only – Single Patient Use Only – Not for Re-Sale or Re-Distribution – Do Not Reuse.
CONTRAINDICATIONS
The use of this device system is contraindicated if the individual has synovial pseudarthrosis.
WARNINGS
Utilization of this stimulator allows full weight bearing on the casted extremity unless gross motion (greater than 5 degrees in any plane) at the nonunion site is present. In such a case, weight bearing is not advised and should not be permitted as this may compromise the effectiveness of the treatment. The safety and effectiveness of the use of the device on individuals lacking skeletal maturity has not been established. Animal safety studies indicate that the device does not interfere with the normal intrinsic activity of the heart. However, the stimulator does interfere with the operation of certain pacemakers. The concomitant use of the device and a pacemaker must be assessed on an individual basis, prior to use (such as with an electrocardiogram).
PRECAUTIONS
Although laboratory teratological studies performed with this device demonstrate no adverse findings, the safety of this device used during pregnancy and nursing in humans has not been established.
PATIENTS
If you have additional questions regarding your doctor’s instructions in using this device, or you are experiencing any type of adverse reaction with the use of this device, contact your doctor immediately. For further product information, refer to the Patient Manual PN1067800-00 or contact EBI®️ Customer Care at 800-526-2579.
References:
- Brighton CT, Pollack SR. Treatment of recalcitrant non-union with a capacitively coupled electrical field. A preliminary report. J Bone Joint Surg Am. 1985;67(4):577-585.
+ The original clinical study which led to PMA approval in 1986 yielded an overall effectiveness/success rate of 72.5% SS&ED.

