Product Portfolio
As the pioneer of electrobiology innovations, EBI has been leading the industry for almost half a century with the largest portfolio of clinically proven, safe and effective implantable and non-invasive bone growth stimulation solutions to support bone regeneration and healing outcomes. Explore our comprehensive portfolio of bone growth stimulation solutions.
NON-INVASIVE SOLUTIONS
Biomet® EBI® Bone Healing System

The Biomet® EBI® Bone Healing System is the original, innovative, nonsurgical, electrical bone growth stimulation device designed as an adjunctive therapy that promotes bone healing. The Bone Healing System is an industry-leading, first-in-class device with integrated Pulsed Electromagnetic Field (PEMF) technology which is backed by extensive, pre-clinical and clinical data. The Bone Healing System is a proven, safe and effective bone growth stimulation solution that has improved patient outcomes for over 45 years.
Biomet® OrthoPak® Non-invasive Bone Growth Stimulator System
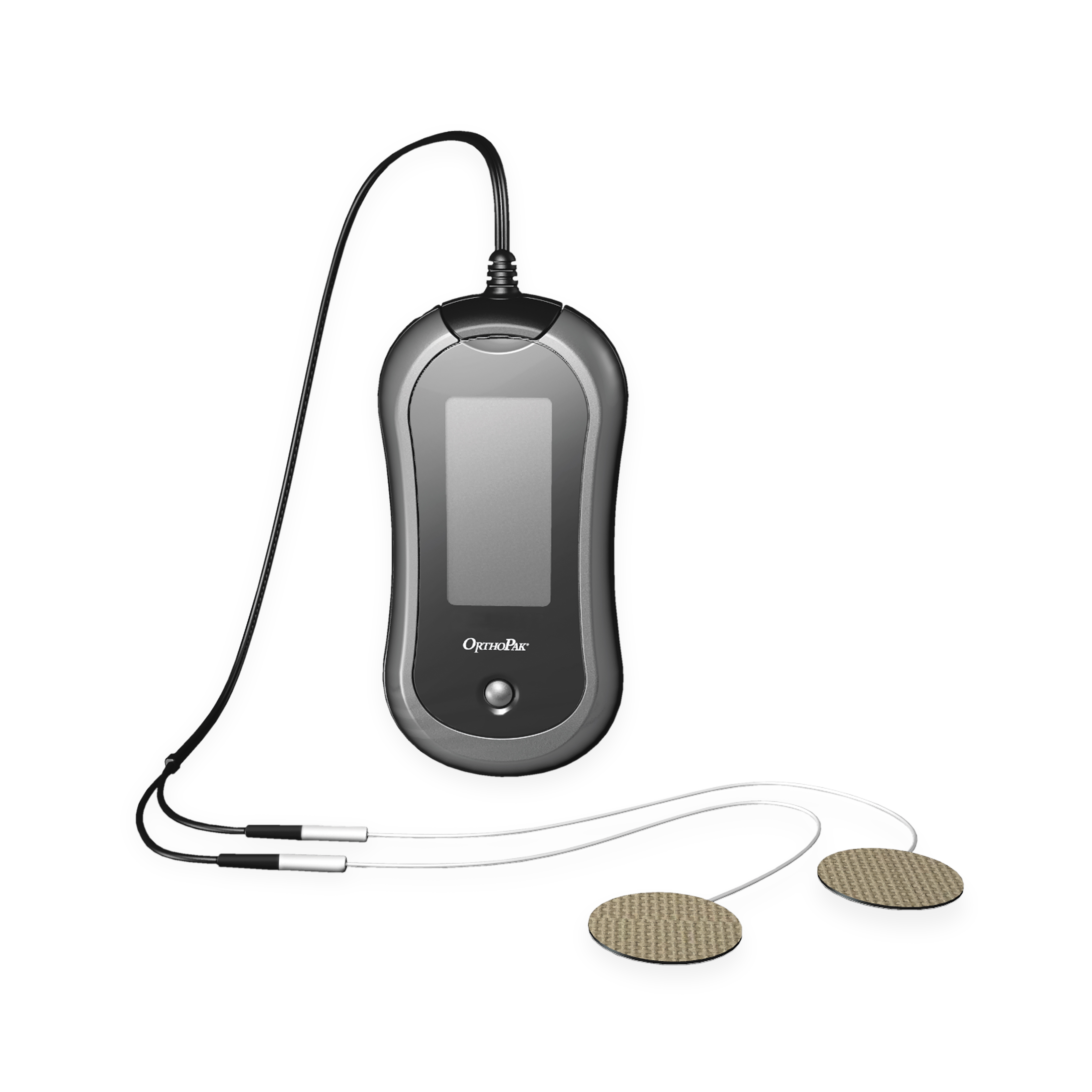
The Biomet® OrthoPak® Non-invasive Bone Growth Stimulator is an innovative, nonsurgical, electrical bone growth stimulation device designed as an adjunctive therapy to aid in the healing of established nonunions. The OrthoPak® stimulator is an industry-leading, first-in-class device with integrated capacitive coupling technology which is backed by extensive, pre-clinical and clinical data. The OrthoPak® device is a proven, safe and effective bone growth stimulation solution that has improved patient outcomes for over 35 years.
Biomet® SpinalPak® Non-invasive Spine Fusion Stimulator System
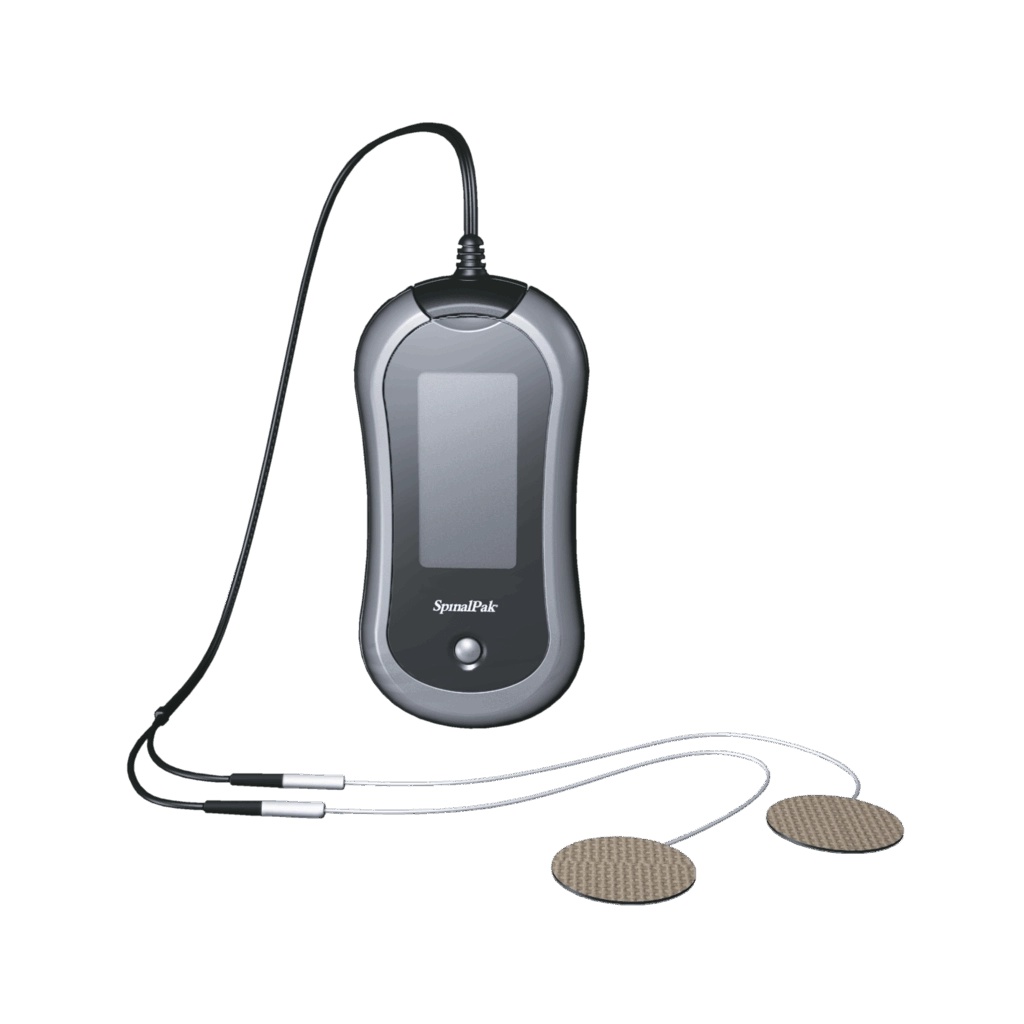
The Biomet® SpinalPak® Non-invasive Spine Fusion Stimulator is an innovative, nonsurgical electrical bone growth stimulation device designed as an adjunctive therapy to aid in the healing of spinal fusions. The SpinalPak® stimulator is an industry-leading, first-in-class device with integrated capacitive coupling technology which is backed by extensive pre-clinical and clinical data. The SpinalPak® device is a proven, safe and effective bone growth stimulation solution that has improved spinal fusion outcomes for over 25 years.
IMPLANTABLE SOLUTIONS
EBI® OsteoGen™ Surgically Implanted Bone Growth Stimulators

The EBI® OsteoGen™ Surgically Implanted Bone Growth Stimulator is an innovative, electrical bone growth stimulation implant designed as an adjunctive therapy to aid in the healing of long bone fracture nonunions. The OsteoGen™ implant is an industry-leading, first-in-class, unique device powered by integrated Direct Current (DC) technology which is backed by extensive, pre-clinical and clinical data. This clinically proven solution offers a quick and simple adjunctive surgical treatment that complements nonunion fracture management to improve the patient’s healing outcome. The OsteoGen™ implant is a proven, safe and effective bone growth stimulation solution that has improved patient outcomes for over 40 years.
SpF® Implantable Spinal Fusion Stimulators

The SpF® Implantable Spinal Fusion Stimulator is an innovative, bone growth stimulation implant designed as an adjunct to aid the healing of posterior lumbar spinal fusion surgery. The SpF® implant is an industry-leading, first-in-class, unique device with integrated Direct Current (DC) technology which is backed by original, pre-clinical and clinical data. The SpF® implant is a proven, safe and effective bone growth stimulation solution that has improved fusion outcomes for over 35 years.
Product Indications, Risk Statements and Disclaimers
The Biomet® EBI® Bone Healing System is indicated for the treatment of fracture nonunions, failed fusions, and congenital pseudarthrosis in the appendicular system.
Contraindicated for nonunion fractures in which a synovial pseudarthrosis exists. Electromagnetic PEMF stimulation is contraindicated for use by patients with implantable pacemakers or defibrillators. The Bone Healing System is not MR safe. Federal Law (U.S.A.) restricts this device to sale by or on the order of a physician. Rx Only. Single Patient Use Only. Do Not Reuse.
The Biomet® OrthoPak® Non-invasive Bone Growth Stimulator is indicated for the treatment of an established nonunion acquired secondary to trauma, excluding vertebrae and all flat bones, where the width of the nonunion defect is less than one-half the width of the bone to be treated.
Contraindicated if the individual has synovial pseudarthrosis. Federal Law (U.S.A.) restricts this device to sale by or on the order of a physician. Rx Only. Single Patient Use Only. Do Not Reuse.
The Biomet® SpinalPak® Non-invasive Spine Fusion Stimulator is a non-invasive spine fusion stimulator indicated as an adjunct electrical treatment to primary lumbar spinal fusion surgery for one or two levels. No known contraindications. Rx Only. Single Patient Use Only. Do Not Reuse.
The EBI® OsteoGen™ Surgically Implanted Bone Growth Stimulators are indicated in the treatment of long bone nonunions.
No known contraindications. Not recommended with the following conditions: pathological fractures due to malignant tumors or active osteomyelitis. Federal Law (U.S.A.) restricts these devices to sale by or on the order of a physician. Rx Only. Single Patient Use Only. Do Not Reuse.
SpF® Implantable Spinal Fusion Stimulators are indicated as a lumbar spinal fusion adjunct to increase the probability of fusion success in one or two levels or three or more levels. Do not use with defibrillators. If the stimulators are used in conjunction with metal internal fixation devices, no metallic part of the stimulator should be allowed to come into contact with the fixation device; this includes minimally invasive surgical-MIS procedures. Any surgical implantation procedure such as minimally invasive surgical-MIS procedures requiring the SpF® cathodes to be disconnected from their corresponding leads prior to or during implantation. Federal Law (U.S.A.) restricts this device to sale by or on the order of a physician. Rx Only. Single Patient Use Only. Do Not Reuse.
Complete prescribing information including full indications, contraindications, warnings and precautions associated with the use of all EBI® Bone Growth Stimulation devices can be found in the labeling documentation on this page. All content herein is protected by copyright, trademarks and other intellectual property rights, as applicable, owned by or licensed to EBI or its affiliates unless otherwise indicated, and must not be redistributed, duplicated or disclosed, in whole or in part, without the express written consent of EBI. REV A 08/25. ©2025 EBI, LLC. All rights reserved.